Protocol: Phenol-chloroform extraction of prokaryotic DNA
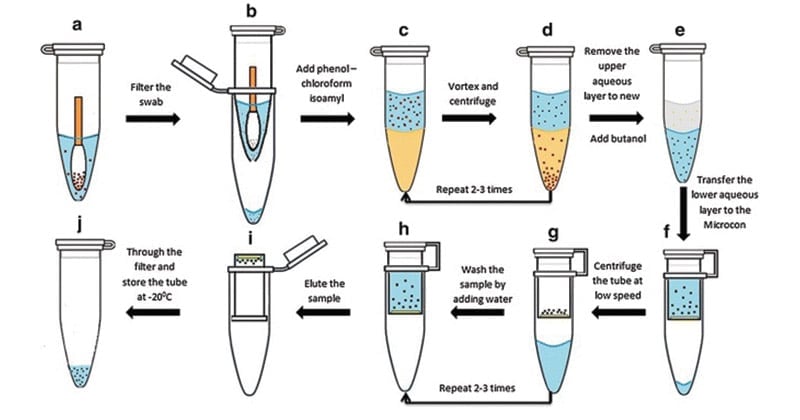

Phenol-chloroform extraction of DNA
A phenol-chloroform extraction is a liquid-liquid extraction. A liquid-liquid extraction is a method that separates mixtures of molecules based on the differential solubilities of the individual molecules in two different immiscible liquids. Liquid-liquid extractions are widely used to isolate RNA, DNA, or proteins.The extraction of nucleic acids involves adding an equal volume of phenol-chloroform to an aqueous solution of lysed cells or homogenized tissue, mixing the two phases, and allowing the phases to separate by centrifugation. Centrifugation of the mixture yields two phases: the lower organic phase and the upper aqueous phase.
Principle
- The organism to be used should be grown in a favorable medium at an optimal temperature, and should be harvested in late log to early stationary phase for maximum yield.
- The genomic DNA isolation needs to separate total DNA from RNA, protein, lipid, etc.
- Initially the cell membranes must be disrupted in order to release the DNA in the extraction buffer. SDS (sodium dodecyl sulphate) is used to disrupt the cell membrane.
- Once cell is disrupted, the endogenous nucleases tend to cause extensive hydrolysis. DNA can be protected from endogenous nucleases by chelating Mg2++ ions using EDTA. Mg2++ ion is considered as a necessary cofactor for action of most of the nucleases.
- Nucleoprotein interactions are disrupted with SDS, phenol or proteinase K.
- Proteinase enzyme is used to degrade the proteins in the disrupted cell soup.
- Phenol and chloroform are used to denature and separate proteins from DNA. Chloroform is also a protein denaturant, which stabilizes the rather unstable boundary between an aqueous phase and pure phenol layer.
- The denatured proteins form a layer at the interface between the aqueous and the organic phases which are removed by centrifugation.
- DNA released from disrupted cells is precipitated by cold absolute ethanol or isopropanol.
Materials and Reagents
- Tris base
- Proteinase K
- Phenolchloroform (1:1)
- 200 proof ethanol
- RNAase
- Ethanol
- SDS
- EDTA
- Tryptone
- Yeast extract
- NaCl
- LB medium
- TE buffer
- Lysis buffer
Equipment
- Tabletop centrifuge
- 1.5 ml Eppendorf tube
- Incubator
Procedure
- Transfer 1.5 ml of the overnight E. coli culture (grown in LB medium) to a 1.5 ml Eppendorf tube and centrifuge at max speed for 1min to pellet the cells.
- Discard the supernatant without disturbing the cell pellet.
- Resuspend the cell pellet in 600 μl lysis buffer and vortex to completely resuspend cell pellet.
- Incubate 1 h at 37 °C.
- Add an equal volume of phenol/chloroform and mix well by inverting the tube until the phases are completely mixed.
- Spin at max speed for 5 min at RT (all spins are performed at RT, unless indicated otherwise). There is a white layer (protein layer) in the aqueous: phenol/chloroform interface.
- Carefully transfer the upper aqueous phase to a new tube by using 1 ml pipetman (to avoid sucking the interface, use 1 ml tip with wider mouth-cut 1 ml tip-mouth about ~2 mm shorter).
- Steps 4-6 can be repeated until the white protein layer disappears.
- To remove phenol, add an equal volume of chloroform to the aqueous layer. Again, mix well by inverting the tube.
- Spin at max speed for 5 min.
- Remove aqueous layer to new tube.
- To precipitate the DNA, add 2.5 or 3 volume of cold 200 proof ethanol (store ethanol at -20 °C freezer) and mix gently (DNA precipitation can be visible).
Note: DNA precipitation may simply diffuse, which is normal. Keep the tube at -20 degree for at least 30 min (the longer the better) and then spin it down (see Steps 15-16). You should see DNA pellet. It looks transparency when it is wet and turns to white when it becomes dry.
- Incubate the tube at -20 °C for 30 min or more.
- Spin at max speed for 15 min at 4 °C.
- Discard the supernatant and rinse the DNA pellet with 1 ml 70% ethanol (stored at RT).
- Spin at max speed for 2 min. Carefully discard the supernatant and air-dry the DNA pellet (tilt the tube a little bit on paper towel). To be faster, dry the tube at 37 °C incubator.
- Resuspend DNA in TE buffer.
Note: Large amounts of RNA will be present in the DNA sample. So, for subsequent reactions, for example, to digest plasmid DNA, add 1-5 μl (1 mg ml-1) RNAase to the digestion solution to completely remove RNA. Or, add RNAase directly to lysis buffer with a final concentration of 1 mg ml-1.
- Isolated Gemonic DNA can be subjected to agarose gel electrophoresis.
Expected Results
- White strands of DNA precipitate.
- On gel, bands with smear patterns from high to low molecular weight range can be seen.
- Most of DNA fragments accumulated at high molecular weight: Not degraded.
- Most of DNA fragments small: DNA might have got degraded.
References
- He, F. (2011). E. coli Genomic DNA Extraction. Bio-protocol Bio101: e97. DOI: 21769/BioProtoc.97.
- Maniatis T., E.F. Fritsch, and J. Sambrook (1982). Molecular Cloning A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Springs Harbor, NY.
- http://www.methodquarterly.com/2014/11/protocol-dna-extractions/
- https://genome.cshlp.org/content/4/6/368.full.pdf
- http://labcenter.dnalc.org/labs/dnaextraction/pdfs/teacher/Lesson%20Plan%20DNA%20Extraction%20from%20Bacteria.pdf
- https://www.sciencedirect.com/topics/neuroscience/dna-extraction
- http://www.srmuniv.ac.in/sites/default/files/files/BT0210%20%20MOLECULAR%20BIOLOGY%20LABORATORYMANUAL.pdf
- http://physiology.med.cornell.edu/faculty/mason/lab/zumbo/files/PHENOL-CHLOROFORM.pdf
- http://www.protocol-online.org/cgi-bin/prot/view_cache.cgi?ID=2052
About Author
Sagar Aryal is a microbiologist and a scientific blogger. He is doing his Ph.D. at the Central Department of Microbiology, Tribhuvan University, Kathmandu, Nepal. He was awarded the DAAD Research Grant to conduct part of his Ph.D. research work for two years (2019-2021) at Helmholtz-Institute for Pharmaceutical Research Saarland (HIPS), Saarbrucken, Germany. Sagar is interested in research on actinobacteria, myxobacteria, and natural products. He is the Research Head of the Department of Natural Products, Kathmandu Research Institute for Biological Sciences (KRIBS), Lalitpur, Nepal. Sagar has more than ten years of experience in blogging, content writing, and SEO. Sagar was awarded the SfAM Communications Award 2015: Professional Communicator Category from the Society for Applied Microbiology (Now: Applied Microbiology International), Cambridge, United Kingdom (UK). Sagar is also the ASM Young Ambassador to Nepal for the American Society for Microbiology since 2023 onwards.
1 thought on “Protocol: Phenol-chloroform extraction of prokaryotic DNA”
Thanks a lot for this protocol. Could you please share a protocol for the lysis buffer? Thanks Reply
Leave a Comment Cancel reply
Topics / Categories

- Agricultural Microbiology (13)
- Bacteriology (124)
- Basic Microbiology (61)
- Biochemical Test (114)
- Biochemistry (165)
- Bioinformatics (23)
- Biology (209)
- Biotechnology (31)
- Cell Biology (107)
- Culture Media (67)
- Difference Between (89)
- Diseases (32)
- Environmental Microbiology (7)
- Epidemiology (23)
- Food Microbiology (51)
- Genetics (79)
- Human Anatomy and Physiology (75)
- Immunology (115)
- Instrumentation (64)
- Microscopy (27)
- Molecular Biology (78)
- Mycology (33)
- Parasitology (26)
- Pharmacology (14)
- Phycology (2)
- Protocols (9)
- Research Methodology (20)
- Staining (29)
- Syllabus (18)
- Virology (50)
- Mycorrhiza: Definition, Types and Significances
- Marine Ecosystem and its Abiotic and Biotic Components
- Homeostasis: Definition, Types, Examples, Applications
- Neutrophils: Definition, Structure, Count, Range, Functions
- Whole Transcriptome Sequencing (WTS) / Total RNA-Seq




