Drug-induced liver injury
Drug-induced liver injury (DILI) is an adverse reaction to drugs or other xenobiotics that occurs either as a predictable event when an individual is exposed to toxic doses of some compounds or as an unpredictable event with many drugs in common use. Drugs can be harmful to the liver in susceptible individuals owing to genetic and environmental risk factors. These risk factors modify hepatic metabolism and excretion of the DILI-causative agent leading to cellular stress, cell death, activation of an adaptive immune response and a failure to adapt, with progression to overt liver injury. Idiosyncratic DILI is a relative rare hepatic disorder but can be severe and, in some cases, fatal, presenting with a variety of phenotypes, which mimic other hepatic diseases. The diagnosis of DILI relies on the exclusion of other aetiologies of liver disease as specific biomarkers are still lacking. Clinical scales such as CIOMS/RUCAM can support the diagnostic process but need refinement. A number of clinical variables, validated in prospective cohorts, can be used to predict a more severe DILI outcome. Although no pharmacological therapy has been adequately tested in randomized clinical trials, corticosteroids can be useful, particularly in the emergent form of DILI related to immune-checkpoint inhibitors in patients with cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
133,45 € per year
only 133,45 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Managing the challenge of drug-induced liver injury: a roadmap for the development and deployment of preclinical predictive models
Article 20 November 2019
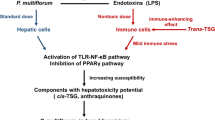
The hepatotoxicity of Polygonum multiflorum: The emerging role of the immune-mediated liver injury
Article 02 March 2020
Performance of preclinical models in predicting drug-induced liver injury in humans: a systematic review
Article Open access 18 March 2021
References
- Chen, M., Suzuki, A., Borlak, J., Andrade, R. J. & Lucena, M. I. Drug-induced liver injury: interactions between drug properties and host factors. J. Hepatol.63, 503–514 (2015). A concept paper addressing drug properties, patient factors and their interplay in DILI. ArticleCASPubMedGoogle Scholar
- Kaplowitz, N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov.4, 489–499 (2005). Key review highlighting important issues and potential mechanism underlying idiosyncratic DILI. ArticleCASPubMedGoogle Scholar
- Reuben, A. et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann. Intern. Med.164, 724–732 (2016). ArticlePubMedPubMed CentralGoogle Scholar
- Donnelly, M. C. et al. Acute liver failure in Scotland: changes in aetiology and outcomes over time (the Scottish Look-Back Study). Aliment. Pharmacol. Ther.45, 833–843 (2017). ArticleCASPubMedGoogle Scholar
- Zimmerman, H. J. & Maddrey, W. C. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology22, 767–773 (1995). ArticleCASPubMedGoogle Scholar
- Watkins, P. B. et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. JAMA296, 87–93 (2006). ArticleCASPubMedGoogle Scholar
- Chen, M., Borlak, J. & Tong, W. High lipophilicity and high daily dose of oral medications are associated with significant risk for drug-induced liver injury. Hepatology58, 388–396 (2013). ArticleCASPubMedGoogle Scholar
- Stevens, J. L. & Baker, T. K. The future of drug safety testing: expanding the view and narrowing the focus. Drug Discov. Today14, 162–167 (2009). ArticlePubMedGoogle Scholar
- Avigan, M. I. & Muñoz, M. A. Perspectives on the regulatory and clinical science of drug-induced liver injury (DILI). en Methods in Pharmacology and Toxicology (eds. Minjun Chen & Yvonne Will) 367-393 (Humana Press, 2018).
- National Institutes of Health. LiverTox: clinical and research information on drug-induced liver injury. Nih.govhttps://livertox.nih.gov (2017).
- Steele, M. A., Burk, R. F. & DesPrez, R. M. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest99, 465–471 (1991). ArticleCASPubMedGoogle Scholar
- Perdices, E. V. et al. Hepatotoxicity associated with statin use: analysis of the cases included in the Spanish Hepatotoxicity Registry. Rev. Esp. enfermedades Dig.106, 246–254 (2014). CASGoogle Scholar
- Björnsson, E. S., Bergmann, O. M., Björnsson, H. K., Kvaran, R. B. & Olafsson, S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology144, 1419–1425 (2013). A population-based study of the incidence and causes of DILI, and the first study to show the risk of DILI with the use of individual agents. ArticlePubMedCASGoogle Scholar
- Björnsson, E., Jacobsen, E. I. & Kalaitzakis, E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J. Hepatol.56, 374–380 (2012). ArticlePubMedCASGoogle Scholar
- Chalasani, N. et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology148, 1340–1352 (2015). ArticlePubMedGoogle Scholar
- Andrade, R. J. et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology129, 512–521 (2005). The primary large publication from the Spanish Hepatotoxicity Registry, with the first large prospective study of patients with DILI. ArticlePubMedGoogle Scholar
- Navarro, V. J. et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology60, 1399–1408 (2014). ArticlePubMedGoogle Scholar
- Andrade, R., Medina-Caliz, I., Gonzalez-Jimenez, A., Garcia-Cortes, M. & Lucena, M. I. Hepatic damage by natural remedies. Semin. Liver Dis.38, 021–040 (2018). ArticleGoogle Scholar
- Suk, K. T. et al. A prospective nationwide study of drug-induced liver injury in Korea. Am. J. Gastroenterol.107, 1380–1387 (2012). ArticlePubMedGoogle Scholar
- Shen, T. et al. Incidence and etiology of drug-induced liver injury in mainland China. Gastroenterology156, 2230–2241.e11 (2019). ArticlePubMedGoogle Scholar
- World Health Organization. Traditional medicine strategy. 2014-2023. 76 Disponible en: https://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/ (Accedido: 30. a enero 2019). (2013).
- Aiso, M. et al. Analysis of 307 cases with drug-induced liver injury between 2010 and 2018 in Japan. Hepatol. Res.49, 105–110 (2019). ArticleCASPubMedGoogle Scholar
- Zhou, Y. et al. Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21,789 patients. Eur. J. Gastroenterol. Hepatol.25, 825–829 (2013). ArticlePubMedGoogle Scholar
- Wai, C.-T. et al. Drug-induced liver injury at an Asian center: a prospective study. Liver Int.27, 465–474 (2007). ArticlePubMedGoogle Scholar
- Devarbhavi, H. Ayurvedic and herbal medicine-induced liver injury: it is time to wake up and take notice. Indian J. Gastroenterol.37, 5–7 (2018). ArticlePubMedGoogle Scholar
- Wang, G.-Q., Deng, Y.-Q. & Hou, F.-Q. Overview of drug-induced liver injury in China. Clin. Liver Dis.4, 26–29 (2014). ArticleGoogle Scholar
- Devarbhavi, H. et al. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis and predictors of mortality. Am. J. Gastroenterol.105, 2396–2404 (2010). ArticlePubMedGoogle Scholar
- World Health Organization. Global tuberculosis report 2017. Who.inthttps://www.who.int/tb/publications/global_report/gtbr2017_main_text.pdf (2017).
- Devarbhavi, H. et al. Drug-induced acute liver failure in children and adults: results of a single-centre study of 128 patients. Liver Int.38, 1322–1329 (2017). ArticlePubMedCASGoogle Scholar
- de Abajo, F. J., Montero, D., Madurga, M. & Rodriguez, L. A. G. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br. J. Clin. Pharmacol.58, 71–80 (2004). ArticlePubMedPubMed CentralGoogle Scholar
- De Valle, M. B., Av Klinteberg, V., Alem, N., Olsson, R. & Björnsson, E. Drug-induced liver injury in a Swedish university hospital out-patient hepatology clinic. Aliment. Pharmacol. Ther.24, 1187–1195 (2006). ArticlePubMedCASGoogle Scholar
- Sgro, C. et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology36, 451–455 (2002). ArticlePubMedGoogle Scholar
- Björnsson, E. & Olsson, R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology42, 481–489 (2005). ArticlePubMedCASGoogle Scholar
- Vega, M. et al. The incidence of drug- and herbal and dietary supplement-induced liver injury: preliminary findings from gastroenterologist-based surveillance in the population of the state of Delaware. Drug Saf.40, 783–787 (2017). The first population-based study in the USA. ArticleCASPubMedPubMed CentralGoogle Scholar
- Goldberg, D. S. et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology148, 1353–61.e3 (2015). ArticlePubMedGoogle Scholar
- Bessone, F. et al. When the creation of a consortium provides useful answers: experience of the Latin American DILI Network (LATINDILIN). Clin. Liver Dis.13, 51–57 (2019). ArticleGoogle Scholar
- Amadi, C. & Orisakwe, O. Herb-induced liver injuries in developing nations: an update. Toxics6, 24 (2018). ArticlePubMed CentralCASGoogle Scholar
- Yuan, L. & Kaplowitz, N. Mechanisms of drug-induced liver injury. Clin. Liver Dis.17, 507–518 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Hagenbuch, B. & Stieger, B. The SLCO (former SLC21) superfamily of transporters. Mol. Aspects Med.34, 396–412 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- Burckhardt, G. & Burckhardt, B. C. in Drug Transporters. Handbook of Experimental Pharmacology, vol 201 (eds. Fromm, M. & Kim, R.) 29–104 (Springer, 2011).
- Kullak-Ublick, G. A. et al. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology120, 525–533 (2001). ArticleCASPubMedGoogle Scholar
- Kovacsics, D., Patik, I. & Özvegy-Laczka, C. The role of organic anion transporting polypeptides in drug absorption, distribution, excretion and drug-drug interactions. Expert Opin. Drug Metab. Toxicol.13, 409–424 (2017). ArticleCASPubMedGoogle Scholar
- Shitara, Y., Hirano, M., Sato, H. & Sugiyama, Y. Gemfibrozil and its glucuronide inhibit the organic anion transporting polypeptide 2 (OATP2/OATP1B1:SLC21A6)-mediated hepatic uptake and CYP2C8-mediated metabolism of cerivastatin: analysis of the mechanism of the clinically relevant drug-drug interaction between cerivastatin and gemfibrozil. J. Pharmacol. Exp. Ther.311, 228–236 (2004). ArticleCASPubMedGoogle Scholar
- Khurana, V., Minocha, M., Pal, D. & Mitra, A. K. Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors. Drug Metabol. Drug Interact.29, 249–259 (2014). CASPubMedPubMed CentralGoogle Scholar
- Ellawatty, W. E. A. et al. Organic cation transporter 1 is responsible for hepatocellular uptake of the tyrosine kinase inhibitor pazopanib. Drug Metab. Dispos.46, 33–40 (2018). ArticleCASPubMedGoogle Scholar
- Food and Drug Administration. Guidance document. In vitro metabolism- and transporter-mediated drug-drug interaction studies guidance for industry. Fda.govhttps://www.fda.gov/regulatory-information/search-fda-guidance-documents/vitro-metabolism-and-transporter-mediated-drug-drug-interaction-studies-guidance-industry (2017).
- Park, B. K. et al. Managing the challenge of chemically reactive metabolites in drug development. Nat. Rev. Drug Discov.10, 292–306 (2011). ArticleCASPubMedGoogle Scholar
- Weaver, R. J. et al. Test systems in drug discovery for hazard identification and risk assessment of human drug-induced liver injury. Expert Opin. Drug Metab. Toxicol.13, 767–782 (2017). ArticleCASPubMedGoogle Scholar
- Daly, A. K. et al. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology132, 272–281 (2007). Candidate gene study highlighting the role of SNPs in genes involved in drug metabolism and excretion. ArticleCASPubMedGoogle Scholar
- Li, Y. et al. In vitro metabolic activation of lumiracoxib in rat and human liver preparations. Drug Metab. Dispos.36, 469–473 (2008). ArticleCASPubMedGoogle Scholar
- He, K. et al. Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. Drug Metab. Dispos.32, 639–646 (2004). ArticleCASPubMedGoogle Scholar
- Kullak-Ublick, G. A. et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut66, 1154–1164 (2017). A state-of-the-art review highlighting recent advances in DILI diagnosis. ArticleCASPubMedGoogle Scholar
- Stieger, B., Fattinger, K., Madon, J., Kullak-Ublick, G. A. & Meier, P. J. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (BSEP) of rat liver. Gastroenterology118, 422–430 (2000). ArticleCASPubMedGoogle Scholar
- Krähenbühl, S., Talos, C., Fischer, S. & Reichen, J. Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology19, 471–479 (1994). PubMedGoogle Scholar
- Tujios, S. & Fontana, R. J. Mechanisms of drug-induced liver injury: from bedside to bench. Nat. Rev. Gastroenterol. Hepatol.8, 202–211 (2011). ArticleCASPubMedGoogle Scholar
- Fattinger, K. et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin. Pharmacol. Ther.69, 223–231 (2001). ArticleCASPubMedGoogle Scholar
- Morgan, R. E. et al. Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol. Sci.118, 485–500 (2010). ArticleCASPubMedGoogle Scholar
- Dawson, S., Stahl, S., Paul, N., Barber, J. & Kenna, J. G. In vitro inhibition of the bile salt export pump correlates with risk of cholestatic drug-induced liver injury in humans. Drug Metab. Dispos.40, 130–138 (2012). ArticleCASPubMedGoogle Scholar
- Funk, C., Ponelle, C., Scheuermann, G. & Pantze, M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol. Pharmacol.59, 627–635 (2001). ArticleCASPubMedGoogle Scholar
- Aleo, M. D. et al. Human drug-induced liver injury severity is highly associated with dual inhibition of liver mitochondrial function and bile salt export pump. Hepatology60, 1015–1022 (2014). ArticleCASPubMedGoogle Scholar
- Keppler, D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb. Exp. Pharmacol.201, 299–323 (2011). ArticleCASGoogle Scholar
- Choi, J. H. et al. MRP2 haplotypes confer differential susceptibility to toxic liver injury. Pharmacogenet. Genomics17, 403–415 (2007). ArticleCASPubMedGoogle Scholar
- Ulzurrun, E. et al. Role of chemical structures and the 1331T>C bile salt export pump polymorphism in idiosyncratic drug-induced liver injury. Liver Int.33, 1378–1385 (2013). ArticleCASPubMedGoogle Scholar
- Miura, Y. et al. Sunitinib-induced severe toxicities in a Japanese patient with the ABCG2 421 AA genotype. BMC Cancer14, 964 (2014). ArticlePubMedPubMed CentralGoogle Scholar
- Lang, C. et al. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet. Genomics17, 47–60 (2007). ArticleCASPubMedGoogle Scholar
- Yoshikado, T. et al. Itraconazole-induced cholestasis: involvement of the inhibition of bile canalicular phospholipid translocator MDR3/ABCB4. Mol. Pharmacol.79, 241–250 (2011). ArticleCASPubMedGoogle Scholar
- Win, S., Than, T. A., Min, R. W. M., Aghajan, M. & Kaplowitz, N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology63, 1987–2003 (2016). ArticleCASPubMedGoogle Scholar
- Iorga, A., Dara, L. & Kaplowitz, N. Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int. J. Mol. Sci.18, 1018 (2017). ArticlePubMed CentralCASGoogle Scholar
- Roth, R. A., Maiuri, A. R. & Ganey, P. E. Idiosyncratic drug-induced liver injury: is drug-cytokine interaction the linchpin? J. Pharmacol. Exp. Ther.360, 461–470 (2017). ArticlePubMedCASGoogle Scholar
- Jaeschke, H., Williams, C. D., Ramachandran, A. & Bajt, M. L. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int.32, 8–20 (2012). ArticleCASPubMedGoogle Scholar
- Jaeschke, H. Innate immunity and acetaminophen-induced liver injury: why so many controversies? Hepatology48, 699–701 (2008). ArticlePubMedGoogle Scholar
- Dara, L. et al. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology62, 1847–1857 (2015). ArticleCASPubMedGoogle Scholar
- Luedde, T., Kaplowitz, N. & Schwabe, R. F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology147, 765–783.e4 (2014). ArticleCASPubMedGoogle Scholar
- Yip, L. Y. et al. The liver-gut microbiota axis modulates hepatotoxicity of tacrine in the rat. Hepatology67, 282–295 (2018). ArticleCASPubMedGoogle Scholar
- Gong, S. et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J. Hepatol.69, 51–59 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Monshi, M. M. et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology57, 727–739 (2013). ArticleCASPubMedGoogle Scholar
- Grove, J. I. & Aithal, G. P. Human leukocyte antigen genetic risk factors of drug-induced liver toxicology. Expert Opin. Drug Metab. Toxicol.11, 395–409 (2015). ArticleCASPubMedGoogle Scholar
- Kaplowitz, N. Avoiding idiosyncratic DILI: two is better than one. Hepatology58, 15–17 (2013). ArticlePubMedGoogle Scholar
- Light, D. S., Aleo, M. D. & Kenna, J. G. in Drug-Induced Liver Toxicity. Methods in Pharmacology and Toxicology (eds Chen, M. & Will, Y.) 345–364 (Humana Press, 2018).
- Kenna, J. G. et al. Can bile salt export pump inhibition testing in drug discovery and development reduce liver injury risk? an international transporter consortium perspective. Clin. Pharmacol. Ther.104, 916–932 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Uetrecht, J. & Kaplowitz, N. Inhibition of immune tolerance unmasks drug-induced allergic hepatitis. Hepatology62, 346–348 (2015). ArticlePubMedGoogle Scholar
- Russmann, S., Jetter, A. & Kullak-Ublick, G. A. Pharmacogenetics of drug-induced liver injury. Hepatology52, 748–761 (2010). ArticleCASPubMedGoogle Scholar
- Institute of Medicine. Exploring the Biological Contributions to Human Health: Does Sex Matter? (National Academies Press, 2001).
- Mennecozzi, M., Landesmann, B., Palosaari, T., Harris, G. & Whelan, M. Sex differences in liver toxicity—do female and male human primary hepatocytes react differently to toxicants in vitro? PLoS One10, e0122786 (2015). ArticlePubMedPubMed CentralCASGoogle Scholar
- Xie, Y. et al. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol.279, 266–274 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Dai, G., He, L., Chou, N. & Wan, Y.-J. Y. Acetaminophen metabolism does not contribute to gender difference in its hepatotoxicity in mouse. Toxicol. Sci.92, 33–41 (2006). ArticleCASPubMedGoogle Scholar
- Sheng, Y., Liang, Q., Deng, Z., Ji, L. & Wang, Z. Acetaminophen induced gender-dependent liver injury and the involvement of GCL and GPx. Drug Discov. Ther.7, 78–83 (2013). CASPubMedGoogle Scholar
- Du, K., Williams, C. D., McGill, M. R. & Jaeschke, H. Lower susceptibility of female mice to acetaminophen hepatotoxicity: role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol. Appl. Pharmacol.281, 58–66 (2014). ArticleCASPubMedPubMed CentralGoogle Scholar
- Leibman, D., Smolen, A. & Smolen, T. N. Strain, sex and developmental profiles of cocaine metabolizing enzymes in mice. Pharmacol. Biochem. Behav.37, 161–165 (1990). ArticleCASPubMedGoogle Scholar
- Visalli, T., Turkall, R. & Abdel-Rahman, M. S. Gender differences in cocaine pharmacokinetics in CF-1 mice. Toxicol. Lett.155, 35–40 (2005). ArticleCASPubMedGoogle Scholar
- You, Q., Cheng, L., Reilly, T. P., Wegmann, D. & Ju, C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology44, 1421–1431 (2006). ArticleCASPubMedGoogle Scholar
- Dugan, C. M., Fullerton, A. M., Roth, R. A. & Ganey, P. E. Natural killer cells mediate severe liver injury in a murine model of halothane hepatitis. Toxicol. Sci.120, 507–518 (2011). ArticleCASPubMedPubMed CentralGoogle Scholar
- Toyoda, Y. et al. Mechanism of exacerbative effect of progesterone on drug-induced liver injury. Toxicol. Sci.126, 16–27 (2012). ArticleCASPubMedGoogle Scholar
- Toyoda, Y. et al. Estradiol and progesterone modulate halothane-induced liver injury in mice. Toxicol. Lett.204, 17–24 (2011). ArticleCASPubMedGoogle Scholar
- Cho, J. et al. Sex bias in experimental immune-mediated, drug-induced liver injury in BALB/c mice: suggested roles for Tregs, estrogen, and IL-6. PLoS One8, e61186 (2013). ArticleCASPubMedPubMed CentralGoogle Scholar
- George, N., Chen, M., Yuen, N., Hunt, C. M. & Suzuki, A. Interplay of gender, age and drug properties on reporting frequency of drug-induced liver injury. Regul. Toxicol. Pharmacol.94, 101–107 (2018). ArticleCASPubMedGoogle Scholar
- Hunt, C. M., Yuen, N. A., Stirnadel-Farrant, H. A. & Suzuki, A. Age-related differences in reporting of drug-associated liver injury: data-mining of WHO Safety Report Database. Regul. Toxicol. Pharmacol.70, 519–526 (2014). ArticleCASPubMedGoogle Scholar
- Lucena, M. I. et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology49, 2001–2009 (2009). ArticlePubMedGoogle Scholar
- Suzuki, A. et al. Associations of gender and a proxy of female menopausal status with histological features of drug-induced liver injury. Liver Int.37, 1723–1730 (2017). ArticlePubMedPubMed CentralGoogle Scholar
- Gonzalez-Jimenez, A. et al. The influence of drug properties and host factors on delayed onset of symptoms in drug-induced liver injury. Liver Int.39, 401–410 (2019). CASPubMedGoogle Scholar
- Ortega-Alonso, A., Stephens, C., Lucena, M. I. & Andrade, R. J. Case characterization, clinical features and risk factors in drug-induced liver injury. Int. J. Mol. Sci.17, 714 (2016). ArticlePubMed CentralCASGoogle Scholar
- Kaliyaperumal, K. et al. Pharmacogenomics of drug-induced liver injury (DILI): molecular biology to clinical applications. J. Hepatol.69, 948–957 (2018). An up-to-date review of genetic susceptibility to DILI in a clinical context. ArticleCASPubMedGoogle Scholar
- Devarbhavi, H. & Raj, S. Drug-induced liver injury with skin reactions: drugs and host risk factors, clinical phenotypes and prognosis. Liver Int.39, 802–811 (2019). ArticlePubMedGoogle Scholar
- Devarbhavi, H., Karanth, D., Prasanna, K. S., Adarsh, C. K. & Patil, M. Drug-induced liver injury with hypersensitivity features has a better outcome: a single-center experience of 39 children and adolescents. Hepatology54, 1344–1350 (2011). ArticleCASPubMedGoogle Scholar
- Devarbhavi, H., Raj, S., Joseph, T., Singh, R. & Patil, M. Features and treatment of dapsone-induced hepatitis, based on analysis of 44 cases and literature review. Clin. Gastroenterol. Hepatol.15, 1805–1807 (2017). ArticlePubMedGoogle Scholar
- Kardaun, S. H. et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br. J. Dermatol.156, 609–611 (2007). ArticleCASPubMedGoogle Scholar
- Bastuji-Garin, S. et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch. Dermatol.129, 92–96 (1993). ArticleCASPubMedGoogle Scholar
- Czaja, A. J. Drug-induced autoimmune-like hepatitis. Dig. Dis. Sci.56, 958–976 (2011). ArticleCASPubMedGoogle Scholar
- Devarbhavi, H. et al. Drug-induced liver injury associated with Stevens-Johnson syndrome/toxic epidermal necrolysis: patient characteristics, causes, and outcome in 36 cases. Hepatology63, 993–999 (2016). ArticleCASPubMedGoogle Scholar
- Aithal, G. P. et al. Case definition and phenotype standardization in drug-induced liver injury. Clin. Pharmacol. Ther.89, 806–815 (2011). This paper established international consensus on case definitions and phenotypic charecterization of DILI. ArticleCASPubMedGoogle Scholar
- Senior, J. R. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury–past, present, and future. Clin. Pharmacol. Ther.92, 332–339 (2012). ArticleCASPubMedGoogle Scholar
- Dara, L., Liu, Z.-X. & Kaplowitz, N. Mechanisms of adaptation and progression in idiosyncratic drug induced liver injury, clinical implications. Liver Int.36, 158–165 (2016). A description of various factors that contribute to pathogenesis of idiosyncratic DILI, highlighting the participation of genetic susceptibility due to HLA variants and the potential importance of immune tolerance. ArticlePubMedGoogle Scholar
- Chalasani, N. et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology135, 1924–1934.e4 (2008). ArticlePubMedGoogle Scholar
- Davern, T. J. et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology141, 1665–1672 (2011). ArticlePubMedGoogle Scholar
- Sanjuan-Jimenez, R. et al. Prevalence of hepatitis E markers in Spanish patients with suspected drug-induced liver injury [abstract]. Hepatology66, 423A (2017). Google Scholar
- Dalton, H. R. et al. EASL Clinical Practice Guidelines on hepatitis E virus infection. J. Hepatol.68, 1256–1271 (2018). ArticleGoogle Scholar
- Suzuki, A. et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology54, 931–939 (2011). ArticlePubMedGoogle Scholar
- Foureau, D. M. et al. Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin. Exp. Immunol.180, 40–51 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Kleiner, D. E. et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology59, 661–670 (2014). ArticlePubMedGoogle Scholar
- Bonkovsky, H. L. et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology65, 1267–1277 (2017). ArticleCASPubMedGoogle Scholar
- Andrade, R. J., Robles, M. & Lucena, M. I. Rechallenge in drug-induced liver injury: the attractive hazard. Expert Opin. Drug Saf.8, 709–714 (2009). ArticlePubMedGoogle Scholar
- García-Cortés, M., Stephens, C., Lucena, M. I., Fernández-Castañer, A. & Andrade, R. J. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J. Hepatol.55, 683–691 (2011). A comprehensive review on the shortcomings of the liver-specific CIOMS/RUCAM scale. ArticlePubMedGoogle Scholar
- García-Cortés, M. et al. Evaluation of Naranjo Adverse Drug Reactions Probability Scale in causality assessment of drug-induced liver injury. Aliment. Pharmacol. Ther.27, 780–789 (2008). ArticlePubMedGoogle Scholar
- Danan, G. & Benichou, C. Causality assessment of adverse reactions to drugs—I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J. Clin. Epidemiol.46, 1323–1330 (1993). ArticleCASPubMedGoogle Scholar
- Lucena, M., Camargo, R., Andrade, R. J., Perez-Sanchez, C. J. & Sanchez De La Cuesta, F. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology33, 123–130 (2001). ArticleCASPubMedGoogle Scholar
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: drug-induced liver injury. J Hepatol. 70, 1222-1261 (2019). A comprehensive review of DILI and recommendations for clinical practice.
- Rochon, J. et al. Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology48, 1175–1183 (2008). ArticlePubMedGoogle Scholar
- Navarro, V. J. et al. Liver injury from herbal and dietary supplements. Hepatology65, 363–373 (2017). ArticleCASPubMedGoogle Scholar
- Suzman, D. L., Pelosof, L., Rosenberg, A. & Avigan, M. I. Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int.38, 976–987 (2018). ArticlePubMedGoogle Scholar
- Rockey, D. C. et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology51, 2117–2126 (2010). ArticlePubMedGoogle Scholar
- Hayashi, P. H. Drug-induced liver injury network causality assessment: criteria and experience in the United States. Int. J. Mol. Sci.17, 201 (2016). ArticlePubMedPubMed CentralCASGoogle Scholar
- Hayashi, P. H. et al. Reliability of causality assessment for drug, herbal and dietary supplement hepatotoxicity in the Drug-Induced Liver Injury Network (DILIN). Liver Int.35, 1623–1632 (2015). ArticlePubMedGoogle Scholar
- Takikawa, H. et al. Assessment of 287 Japanese cases of drug induced liver injury by the diagnostic scale of the International Consensus Meeting. Hepatol. Res.27, 192–195 (2003). ArticlePubMedGoogle Scholar
- Whritenour, J. et al. Development of a modified lymphocyte transformation test for diagnosing drug-induced liver injury associated with an adaptive immune response. J. Immunotoxicol.14, 31–38 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Benesic, A. et al. Development and validation of a test to identify drugs that cause idiosyncratic drug-induced liver injury. Clin. Gastroenterol. Hepatol.16, 1488–1494.e5 (2018). ArticleCASPubMedGoogle Scholar
- Danan, G. & Teschke, R. RUCAM in drug and herb induced liver injury: the update. Int. J. Mol. Sci.17, 14 (2015). ArticlePubMed CentralCASGoogle Scholar
- Church, R. J. & Watkins, P. B. The transformation in biomarker detection and management of drug-induced liver injury. Liver Int.37, 1582–1590 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Food and Drug Administration. FDA letter of support for drug-induced liver injury (DILI) biomarkers. Fda.govhttps://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM517355.pdf (2016).
- Hu, J. et al. MiR-122 in hepatic function and liver diseases. Protein Cell3, 364–371 (2012). ArticleCASPubMedPubMed CentralGoogle Scholar
- Church, R. J. et al. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: an international collaborative effort. Hepatology69, 760–763 (2019). ArticleCASPubMedGoogle Scholar
- Rivkin, M. et al. Inflammation-induced expression and secretion of microRNA 122 leads to reduced blood levels of kidney-derived erythropoietin and anemia. Gastroenterology151, 999–1010.e3 (2016). ArticleCASPubMedGoogle Scholar
- Chai, C. et al. Metabolic circuit involving free fatty acids, microRNA 122, and triglyceride synthesis in liver and muscle tissues. Gastroenterology153, 1404–1415 (2017). ArticleCASPubMedGoogle Scholar
- Church, R. J. & Watkins, P. B. Reply. Hepatology67, 2481–2482 (2018). ArticlePubMedGoogle Scholar
- Schmidt, E. S. & Schmidt, F. W. Glutamate dehydrogenase: biochemical and clinical aspects of an interesting enzyme. Clin. Chim. Acta.173, 43–55 (1988). ArticleCASPubMedGoogle Scholar
- Russo, M. W. et al. Profiles of miRNAs in serum in severe acute drug induced liver injury and their prognostic significance. Liver Int.37, 757–764 (2017). ArticleCASPubMedGoogle Scholar
- Steuerwald, N. M. et al. Profiles of serum cytokines in acute drug-induced liver injury and their prognostic significance. PLoS One8, e81974 (2013). ArticlePubMedPubMed CentralCASGoogle Scholar
- Zimmerman, H. J. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. (Lippincott Williams and Wilkins, 1999).
- Temple, R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol. Drug Saf.15, 241–243 (2006). ArticlePubMedGoogle Scholar
- Senior, J. R. Evolution of the Food and Drug Administration approach to liver safety assessment for new drugs: current status and challenges. Drug Saf.37 (Suppl 1), S9–S17 (2014). An important paper from the regulatory perspective of the risk of DILI with new compounds. ArticlePubMedCASGoogle Scholar
- Robles–Diaz, M. et al. Use of Hy’s law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology147, 109–118 (2014). ArticlePubMedCASGoogle Scholar
- Björnsson, E., Nordlinder, H. & Olsson, R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J. Hepatol.44, 791–797 (2006). ArticlePubMedCASGoogle Scholar
- Pachkoria, K. et al. Analysis of IL-10, IL-4 and TNF-alpha polymorphisms in drug-induced liver injury (DILI) and its outcome. J. Hepatol.49, 107–114 (2008). ArticleCASPubMedGoogle Scholar
- Medina-Caliz, I. et al. Definition and risk factors for chronicity following acute idiosyncratic drug-induced liver injury. J. Hepatol.65, 532–542 (2016). ArticleCASPubMedPubMed CentralGoogle Scholar
- Hayashi, P. H. et al. Death and liver transplantation within 2 years of onset of drug-induced liver injury. Hepatology66, 1275–1285 (2017). ArticleCASPubMedGoogle Scholar
- García-Cortés, M., Ortega-Alonso, A., Lucena, M. I. & Andrade, R. J. Drug-induced liver injury: a safety review. Expert Opin. Drug Saf.17, 795–804 (2018). ArticlePubMedCASGoogle Scholar
- Regev, A. Drug-induced liver injury and drug development: industry perspective. Semin. Liver Dis.34, 227–239 (2014). ArticleCASPubMedGoogle Scholar
- Food and Drug Administration. Guidance document. Drug-induced liver injury: premarketing clinical evaluation. Fda.govhttps://www.fda.gov/downloads/Guidances/UCM174090.pdf (2009). A key guidance document for industry on detecting, assessing and managing liver toxicity in clinical trials.
- Newsome, P. N. et al. Guidelines on the management of abnormal liver blood tests. Gut67, 6–19 (2018). ArticlePubMedGoogle Scholar
- Ozer, J., Ratner, M., Shaw, M., Bailey, W. & Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology245, 194–205 (2008). ArticleCASPubMedGoogle Scholar
- Hunt, C. M., Forster, J. K., Papay, J. I. & Stirnadel, H. A. Evidence-based liver chemistry monitoring in drug development. Pharmaceut. Med.23, 149–158 (2009). A retrospective analysis of time-to-onset, incidence, phenotype and severity of liver injury in clinical trials with recommendation on monitoring intervals by development stage. Google Scholar
- Lee, W. M. Drug-induced hepatotoxicity. N. Engl. J. Med.349, 474–485 (2003). ArticleCASPubMedGoogle Scholar
- Devarbhavi, H. An update on drug-induced liver Injury. J. Clin. Exp. Hepatol.2, 247–259 (2012). ArticlePubMedPubMed CentralGoogle Scholar
- Kaplowitz, N. Rules and laws of drug hepatotoxicity. Pharmacoepidemiol. Drug Saf.15, 231–233 (2006). ArticleCASPubMedGoogle Scholar
- Guo, T., Senior, J., Gelperin, K. & Food and Drug Administration. How a SAS/IntrNet tool was created at the FDA for the detection of potential drug-induced liver injury using data with CDISC standard. Lexjansen.comhttps://www.lexjansen.com/wuss/2009/cdi/CDI-Guo.pdf (2008).
- Watkins, P. B. et al. Evaluation of drug-induced serious hepatotoxicity (eDISH): application of this data organization approach to phase III clinical trials of rivaroxaban after total hip or knee replacement surgery. Drug Saf.34, 243–252 (2011). ArticlePubMedGoogle Scholar
- Merz, M., Lee, K. R., Kullak-Ublick, G. A., Brueckner, A. & Watkins, P. B. Methodology to assess clinical liver safety data. Drug Saf.37, 33–45 (2014). ArticleCASPubMed CentralGoogle Scholar
- Graham, D. J., Green, L., Senior, J. R. & Nourjah, P. Troglitazone-induced liver failure: a case study. Am. J. Med.114, 299–306 (2003). ArticlePubMedGoogle Scholar
- Graham, D. J., Drinkard, C. R. & Shatin, D. Incidence of idiopathic acute liver failure and hospitalized liver injury in patients treated with troglitazone. Am. J. Gastroenterol.98, 175–179 (2003). ArticleCASPubMedGoogle Scholar
- Graham, D. J., Drinkard, C. R., Shatin, D., Tsong, Y. & Burgess, M. J. Liver enzyme monitoring in patients treated with troglitazone. JAMA286, 831–833 (2001). ArticleCASPubMedGoogle Scholar
- Chalasani, N. P. et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am. J. Gastroenterol.109, 950–966 (2014). The first guidelines for the diagnosis and management of DILI. ArticlePubMedGoogle Scholar
- Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer5, 95 (2017). ArticleCASPubMedPubMed CentralGoogle Scholar
- Abu-Sbeih, H., Styskel, B., Blechazz, B., Chalasani, N. & Miller, E. Clinically significant hepatotoxicity due to immune checkpoint inhibitors is rare but leads to treatment discontinuations in high proportion. Hepatology68, 25A (2018). Google Scholar
- De Martin, E. et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J. Hepatol.68, 1181–1190 (2018). ArticlePubMedCASGoogle Scholar
- Gauci, M.-L. et al. Immune-related hepatitis with immunotherapy: are corticosteroids always needed? J. Hepatol.69, 548–550 (2018). ArticlePubMedGoogle Scholar
- Lee, W. M. et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology137, 856–864 (2009). ArticleCASPubMedGoogle Scholar
- Butt, T. F., Cox, A. R., Oyebode, J. R. & Ferner, R. E. Internet accounts of serious adverse drug reactions: a study of experiences of Stevens-Johnson syndrome and toxic epidermal necrolysis. Drug Saf.35, 1159–1170 (2012). ArticlePubMedGoogle Scholar
- Kowski, A. B. et al. Specific adverse effects of antiepileptic drugs–a true-to-life monotherapy study. Epilepsy Behav.54, 150–157 (2016). ArticlePubMedGoogle Scholar
- Ware, J. E. & Sherbourne, C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care30, 473–483 (1992). ArticlePubMedGoogle Scholar
- Horne, R., Weinman, J. & Hankins, M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Heal.14, 1–24 (1999). ArticleGoogle Scholar
- Suh, J. I. et al. Anxiety and depression propensities in patients with acute toxic liver injury. World J. Gastroenterol.19, 9069–9076 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Fontana, R. J. et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am. J. Gastroenterol.110, 1450–1459 (2015). ArticleCASPubMedPubMed CentralGoogle Scholar
- Ostapowicz, G. et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med.137, 947–954 (2002). ArticlePubMedGoogle Scholar
- Rangnekar, A. S. et al. Quality of life is significantly impaired in long-term survivors of acute liver failure and particularly in acetaminophen-overdose patients. Liver Transpl.19, 991–1000 (2013). ArticlePubMedPubMed CentralGoogle Scholar
- Rzouq, F. S. et al. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am. J. Med. Sci.340, 89–93 (2010). ArticlePubMedGoogle Scholar
- Watkins, P. B. The DILI-sim initiative: insights into hepatotoxicity. mechanisms and biomarker interpretation. Clin. Transl. Sci.12, 122–129 (2019). This is a review of a 15 year public–private partnership that has been using quantitative systems toxicology approaches to understand and predict the likelihood that a new drug candidate will cause DILI. The review focuses on specific advances in mechanistic understanding that have evolved from this consortium. ArticlePubMedPubMed CentralGoogle Scholar
- Aithal, G. P. Pharmacogenetic testing in idiosyncratic drug-induced liver injury: current role in clinical practice. Liver Int.35, 1801–1808 (2015). ArticleCASPubMedGoogle Scholar
- Alvarez, F. et al. International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol.31, 929–938 (1999). ArticleCASPubMedGoogle Scholar
- Kowalec, K. et al. Common variation near IRF6 is associated with IFN-β-induced liver injury in multiple sclerosis. Nat. Genet.50, 1081–1085 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Cirulli, E. T. et al. A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology156, 1707–1716.e2 (2019). ArticleCASPubMedGoogle Scholar
- Kozyra, M., Ingelman-Sundberg, M. & Lauschke, V. M. Rare genetic variants in cellular transporters, metabolic enzymes, and nuclear receptors can be important determinants of interindividual differences in drug response. Genet. Med.19, 20–29 (2017). ArticleCASPubMedGoogle Scholar
- Khera, A. V. et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet.50, 1219–1224 (2018). ArticleCASPubMedPubMed CentralGoogle Scholar
- Metushi, I. G., Hayes, M. A. & Uetrecht, J. Treatment of PD-1(-/-) mice with amodiaquine and anti-CTLA4 leads to liver injury similar to idiosyncratic liver injury in patients. Hepatology61, 1332–1342 (2015). ArticleCASPubMedGoogle Scholar
- Fontana, R. J. et al. Idiosyncratic drug-induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology147, 96–108 (2014). ArticleCASPubMedGoogle Scholar
- Jaeschke, H. & McGill, M. R. Serum glutamate dehydrogenase—biomarker for liver cell death or mitochondrial dysfunction? Toxicol. Sci.134, 221–222 (2013). ArticleCASPubMedGoogle Scholar
- Francis, B. et al. Temporary removal: reference intervals for putative biomarkers of drug-induced liver injury and liver regeneration in healthy human volunteers. J. Hepatol.https://doi.org/10.1016/j.jhep.2018.04.024 (2018). Google Scholar
- Suzuki, A. et al. Comedications alter drug-induced liver injury reporting frequency: data mining in the WHO VigiBase TM . Regul. Toxicol. Pharmacol.72, 481–490 (2015). ArticlePubMedPubMed CentralGoogle Scholar
- Suzuki, A. et al. Co-medications that modulate liver injury and repair influence clinical outcome of acetaminophen-associated liver injury. Clin. Gastroenterol. Hepatol.7, 882–888 (2009). ArticlePubMedGoogle Scholar
- Bianchi, I., Lleo, A., Gershwin, M. E. & Invernizzi, P. The X chromosome and immune associated genes. J. Autoimmun.38, J187–J192 (2012). ArticleCASPubMedGoogle Scholar
- Gianesin, K. et al. Premature aging and immune senescence in HIV-infected children. AIDS30, 1363–1373 (2016). ArticleCASPubMedGoogle Scholar
- Nakajima, T. et al. Premature telomere shortening and impaired regenerative response in hepatocytes of individuals with NAFLD. Liver Int.26, 23–31 (2006). ArticleCASPubMedGoogle Scholar
Acknowledgements
We acknowledge the support of the European Cooperation in Science & Technology (COST) Action CA17112 Prospective European Drug-Induced Liver Injury Network. R.J.A., N.C., E.S.B., A.S., G.A.K.-U, H.D., M.M., M.I.L. and G.P.A. are members of COST Action CA17112.









